Background: Hodgkin lymphoma (HL) is a rare lymphoma that often affects young people, with a median age of diagnosis of 39. While the 5-year PFS rate for patients after front-line chemotherapy is 94.6%, those who relapse have a 50% to 60% rate of cure (Alinari and Blum, Blood 2016). We recently discovered that increased abdominal visceral fat normalized to subcutaneous fat (rVFA) is associated with poorer outcomes in females, but not males, with diffuse large B-cell lymphoma (DLBCL). (Teja e tal ASH 2018) Interestingly, increased body mass index overall has been found to be associated with favorable prognosis in DLBCL (Carson et al., Journal of Clinical Oncology 2012). The correlation between these findings and elevated fasting blood glucose further supports a relationship between metabolism and prognosis in lymphoma. Since HL patients are generally younger and healthier than DLBCL patients at diagnosis, we sought to determine if rVFA and other metabolic markers had similar prognostic value in relapsed/refractory HL patients.
Methods: We conducted a retrospective study of 95 consecutively treated relapsed/refractory HL patients treated at Washington University in St. Louis, who presented with first relapse between 2004 and 2018. We recorded baseline clinical risk factors such as sex, age, BMI, IPI, diabetes status, extranodal sites at relapse, and duration of response to initial therapy. Treatment and response to therapy by PET/CT were also recorded.
In 80 patients, pre-treatment CT images were available to determine areas of subcutaneous and visceral fat at the level of the umbilicus. These values were normalized to total fat to calculate rVFA (Nguyen Radiology, 2018). Thresholds for risk stratification and sex differences were obtained using Cutoff Finder (Budczies PLOS One 2012). Data was analyzed with SPSS.
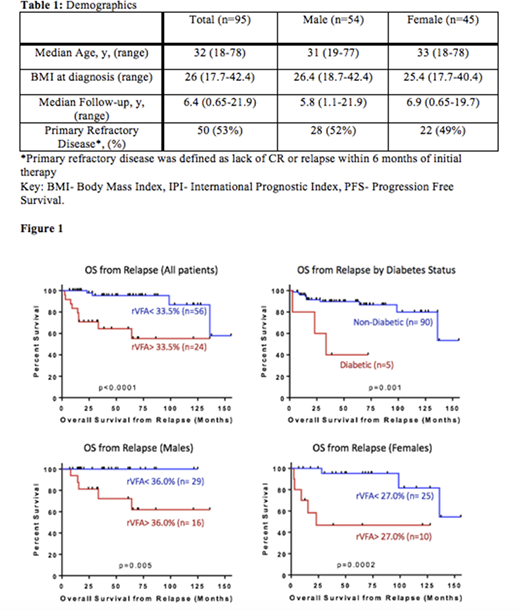
Results: Ninety-five patients were eligible for analysis (54M, 41F) and 50 (53%) had refractory disease to initial therapy. (Table 1) The overall response rate to salvage therapy was 77%. There was no significant difference in overall survival (OS) (p = 0.55) or progression-free survival (PFS) (p = 0.49) between males and females in our study. Patients with higher BMI at diagnosis had a trend towards inferior OS and PFS after relapse. Those with BMI<25 had a 5-year PFS and OS of 71% and 90% compared to the BMI ≥25 group with PFS and OS of 58% and 84% respectively (p=0.09). The five patients with type II diabetes had a significantly lower 5-year OS rate of 40% compared to 90% for non-diabetic patients (p<0.001). (Figure 1)
Eighty patients had CT images available for rVFA analysis (45M, 35F). Both males and females had a median rVFA of 28%. Patients with rVFA greater than 34% had inferior OS after relapse (HR= 8.85, 95% CI: 2.38-32.93, p<0.001). When controlled for sex, rVFA threshold values above 36% and 27% predicted worse outcomes for males and females respectively (HR=2x109, 95% CI: 0- Inf, p=0.0048 for 0.36 threshold; HR= 8.86, 95% CI: 1.7- 46.16, p=0.0018 for 0.27 threshold). (Figure 1)
Conclusion: In this cohort, elevated rVFA was associated with inferior OS in all patients with relapsed/refractory HL, which is consistent with our findings in DLBCL as well as other solid tumors. Our results also suggest an association between diabetes and HL prognosis. Given the association of increased BMI, rVFA, diabetes and poorer outcomes in our cohort, there may be an association between a patient's metabolism and prognosis in Hodgkin lymphoma. Further studies should be conducted to examine the relationship between metabolism and cancer prognosis in a larger cohort.
Bartlett:Seattle Genetics: Consultancy, Research Funding; Immune Design: Research Funding; Forty Seven: Research Funding; Millennium: Research Funding; Merck: Research Funding; BTG: Consultancy; Roche/Genentech: Consultancy, Research Funding; BMS/Celgene: Research Funding; Pharmacyclics: Research Funding; Janssen: Research Funding; Acerta: Consultancy; Kite, a Gilead Company: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Autolus: Research Funding; ADC Therapeutics: Consultancy; Affimed Therapeutics: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ippolito:Vital Images, Inc: Research Funding. Mehta-Shah:Karyopharm Therapeutics: Consultancy; C4 Therapeutics: Consultancy; Verastem: Research Funding; Celgene: Research Funding; Genetech/Roche: Research Funding; Corvus: Research Funding; Bristol Myers-Squibb: Research Funding; Kyowa Hakko Kirin: Consultancy; Innate Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.